Understanding Low Endotoxin Recovery
Low Endotoxin Recovery (LER) studies are essential for analysing drug products that have been intentionally exposed to endotoxins to determine their potential to obscure these harmful substances.
At the 2013 Parenteral Drug Association Annual Meeting in Orlando, Florida, the phenomenon of endotoxin masking in spiked samples was highlighted, indicating the complexity of detecting endotoxins in products containing certain inhibitory substances like proteins, citrates, and phosphates.
As detailed in "Big Pharma Heads to Wickham Micro: A Case Study on Bacterial Endotoxin Testing":
‘Endotoxins are constituents of the bacterial cell wall of Gram-negative bacteria and are released during the break down of Gram-negative bacterial cell membranes. Quality control measures for parenteral drugs require testing for endotoxins as they can illicit an immune response resulting in a pyrogenic reaction when they enter the bloodstream.
Each drug or medical device has a limit specifying the amount of endotoxin that can be safely present. Endotoxin levels that are higher than that limit are dangerous due to their pyrogenic response and at higher doses, toxic effects, so it is vital that all drug compounds and medical devices undergo this safety screening’.
Endotoxins, integral to the cell walls of Gram-negative bacteria, pose significant risks when present in intravenous drugs, potentially triggering severe immune reactions.
The critical nature of these studies lies in their ability to prevent false-negative outcomes in Limulus Amebocyte Lysate (LAL) tests, which could lead to the administration of unsafe drug products to patients, with potentially catastrophic consequences.
Furthermore, conducting these studies is a regulatory requirement, ensuring that new drug developments comply with stringent standards such as the European Pharmacopoeia Section 11.0 2.6.14, USP-NF 2023 Issue 2 <85>, and Japanese Pharmacopeia XVIII 4.01, safeguarding public health and product efficacy.
Low Endotoxin Recovery Case Study
At Wickham Micro, the BET/CC Department conducts LER studies. The initial phase involves preparing a protocol and performing a Method Suitability test to validate the sample product using the most appropriate Kinetic Quantitative method: either the Kinetic Quantitative Turbidimetric Lysate (KQTL) or the Kinetic Quantitative Chromogenic Lysate (KQCL).
Both methods have a detection limit of 0.005 EU/mL. This stage determines the necessary dilution and quantity of the product for the study, ensuring pH levels and other conditions meet Pharmacopoeia standards.
The presence of endotoxin influences temperature and time-dependent recovery, which varies with the endotoxin source, product strength, and formulation buffer.
The preliminary study establishes storage conditions and time points for analysis, with recent studies using storage temperatures of 2-8°C and 20-25°C, and testing at various intervals up to 336 hours. To control for concentration variability, the same endotoxin source is used for both spiking samples and preparing the standard curve.
Low Endotoxin Recovery Testing
In a recent study, three batches of a product were each spiked with a uniform endotoxin level and segmented into three replicates.
These were then stored at 2-8°C and 20-25°C. The testing protocol included baseline endotoxin measurements at T=0, followed by subsequent assessments at 24, 48, 168, and 336 hours to evaluate the influence of time and temperature on endotoxin recovery.
The standardisation of endotoxin sources across samples and controls ensured consistent testing conditions, while the client's provision of the test material's identity, strength, purity, and stability informed the study's parameters.
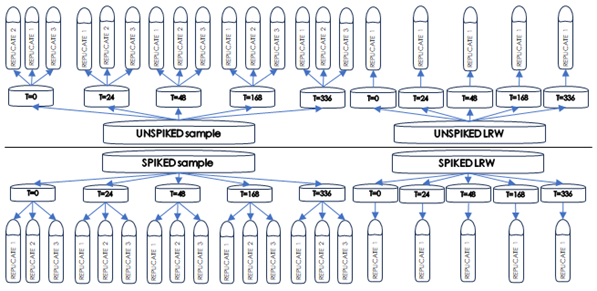
LER Results
The study's initial findings indicated valid test conditions, as evidenced by the control recoveries. However, the endotoxin recovery in spiked samples, particularly those stored at 2-8°C and 20-25°C, showed less than 50% recovery compared to the T=0 baseline within the first 24 hours. This significant reduction suggested a potential LER effect, prompting the client to halt further testing.
Conclusion
LER studies hold paramount importance in the realm of drug safety and regulatory adherence, with the capacity to unveil the interactions between drug products and endotoxins. The LER observed in the Wickham Micro case study highlights the nuanced nature of endotoxin testing.
Notably, the presence of an inhibitory substance within the product sample could be a contributing factor to the LER phenomenon observed, affecting the LAL test's ability to detect spiked endotoxins accurately.
This insight underscores the need for meticulous consideration of sample preparation and testing timelines. Future endeavours might include refining sample preparation protocols and exploring different testing intervals to more precisely capture the dynamics of endotoxin masking.
Such methodological enhancements are crucial for improving the reliability of LAL tests, thereby ensuring the safety of pharmaceutical products and fulfilling stringent regulatory requirements.
Questions or Inquiries?
If you have any questions or need further information about the upcoming webinar on Low Endotoxin Recovery studies, please don't hesitate to reach out. Our dedicated sales team is here to assist you with any inquiries you may have. Contact us at sales@wickhammicro.co.uk, and we'll be more than happy to provide the assistance you need.
We're committed to ensuring that you have all the information and support required to make the most out of this insightful session.
Written By Xavier Martí Cardona, Scientist – Project Lead at the BET/CC Department




